All patients enrolled in the CONFORM Pivotal Trial will receive a LAAO device. One study group will receive the CLAAS AcuFORM Implant, and the other will receive a commercially available implant.
CAUTION: Investigational device. Limited by Federal (or United States) law to investigational use.
Not for sale in any geography.
For more information, please visit: ClinicalTrials.gov (NCT05147792).

Clinical Trial Participants

Up to 1,600 patients will be enrolled in the CONFORM Pivotal Trial across the United States and Europe with participants receiving highly personalized care. Dedicated medical teams will closely monitor each patient’s health and progress, ensuring their well-being remains a top priority.
Patients enrolled in the clinical trial will also benefit from ongoing follow-up for five years after the device implant, highlighting the study’s commitment to comprehensive, long-term care.
How the LAAO Procedure Works
The CLAAS AcuFORM Implant is permanently placed by a physician using a standard minimally invasive procedure.
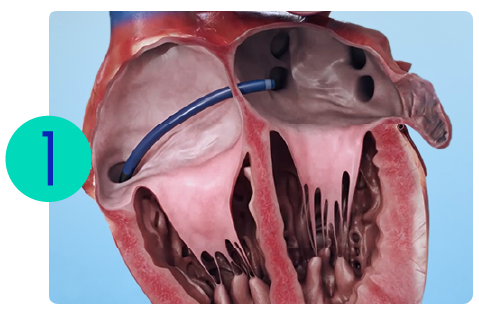
A catheter is placed through a small incision in the groin area and guided through a vein to your heart.

A catheter is positioned at the opening of the LAA.

The CLAAS AcuFORM Implant is passed through the catheter and opens in the LAA.

Once placed, the CLAAS AcuFORM Implant remains in the LAA and with time, heart tissue grows over the device.


